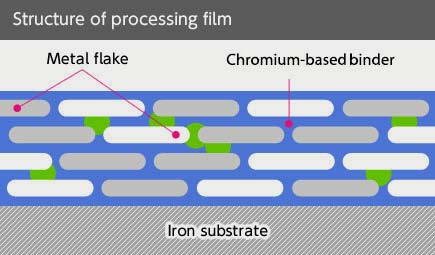
Trivalent Zinc Plating
A process known as zinc plating is frequently used to protect metals such as iron and steel against the relentless forces of corrosion. Zinc plating involves the electrodeposition of a thin coating of zinc metal onto the surface of another metal object, known as a substrate. The zinc coating creates a physical barrier that prevents rust from reaching the underlying metal surface. Zinc is chosen because of its innate ability to fight corrosion. In fact, zinc is often referred to as the "corrosion-prevention workhorse".
The Step-by-Step Zinc Plating Process
Zinc plating is a relatively complex process that requires a high level of expertise. Specialized equipment and machinery is also required, including a rectifier, plating station, ancillary tank for proper dissolution of the zinc anode and a reservoir. Here’s an overview of how a typical zinc plating process works:
Preparing and cleaning the surface — It’s essential to thoroughly clean the surface of the substrate prior to plating. Any debris or contaminants remaining on the surface will prevent proper adhesion of the zinc coating. An alkaline detergent solution is typically used for surface cleaning, which is followed by the application of an acid treatment to remove surface rust. This latter procedure is referred to as pickling.
Preparing the plating solution — Zinc plating requires the immersion of the substrate into a specially formulated electrolyte solution, which is referred to as the plating bath. The bath consists of the zinc metal ionic solution and various chemicals that facilitate plating processes. They also help produce the desired chemical and physical properties of the finished product. Specific types of zinc electrolyte solutions include:
Acid zinc — This is a widely used plating technology known for its high efficiency, fast deposition and superior covering power. However, acid zinc also provides poor throwing power and thickness distribution.
Alkaline zinc — This offers less plating efficiency than acid zinc and a slower electrodeposition rate, but it provides better thickness distribution and ductility.
Choosing the appropriate zinc plating procedure — After solution preparation, the parts are ready for plating. The chosen method could involve Rack Plating, where larger parts are affixed to metal racks which are placed inside the tank containing the plating bath. The parts remain stationary during plating. Barrel plating is normally used for smaller parts — instead of a plating tank, the parts are placed inside a barrel and rotated, which provides a more uniform finish.
Introducing the electrical current — Electroplating is also known as electrodeposition because an electrical current is used to deposit metal ions onto the surface of the substrate. In the case of zinc plating, the substrate serves as the cathode. A DC current originating at the anode is introduced into the bath and flows to the substrate. The zinc ions are then deposited onto the surface. The current flows from the cathode back to the anode to complete the circuit.
Post-treatment procedure — Upon completion of the electrodeposition process, the parts are ready for post-treatment. This normally involves rinsing the parts in water to remove any remaining contaminants and plating bath remnants. In cases of heavy contamination, the parts may need to be rinsed several times. The final step is to thoroughly dry the zinc-plated parts. In situations where additional corrosion protection is required, the application of passivates and sealers can be included in the post-treatment process.
Factors Impacting Zinc Plating Results
There are a variety of factors that can influence the outcome of a zinc plating project, most of which can be effectively managed and controlled by an experienced metal finishing solutions provider. Some factors include:
Current density — The density of the DC current flowing from the anode to the cathode can have a significant impact on the thickness of the zinc coating. The higher the current density, the greater the thickness coating. If the current density exceeds practical limits, a wrinkled substrate surface is likely to result.
Temperature — The temperature of the plating bath will also have a direct impact on the zinc plating outcome. Higher bath temperatures tend to reduce hydrogen diffusion on the cathode and increase the consumption of brighteners and other additives. There is also a close relationship between temperature and current density. When both are increased, the result will be a brighter zinc deposit. When the temperature increases but current density remains unchanged, the formation of larger metallic crystals will occur.
Zinc Plating Applications
Plating with zinc has many industrial applications. Zinc can provide a corrosion-resistant coating on smaller metal parts such as nuts, bolts, screws and fasteners. In general, most hardware parts are coated with zinc. Zinc plating has also gained widespread use in the automotive industry as a means of protecting parts such as brake pipes, brake calipers and power steering components.
Additionally, zinc plating is used in the production of tanks, armored personnel carriers and other heavy military vehicles. Zinc plating can also serve as a protective undercoating prior to painting, as it can promote greater paint adhesion.